近日,广东工业大学环境健康与污染控制研究院、环境科学与工程学院安太成教授团队在大气有机胺对混合态颗粒物贡献机制方面取得最新进展。研究成果以《Contribution of reaction of atmospheric amine with sulfuric acid to mixing particle formation from clay mineral》为题发表在Science of the Total Environment(https://doi.org/10.1016/j.scitotenv.2022.153336)上。论文的第一作者为张维娜副教授,通讯作者为安太成教授。
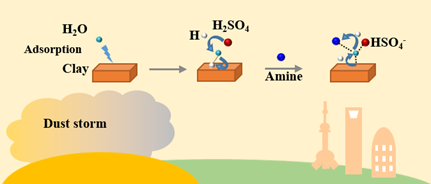
矿物颗粒物是大气气溶胶最主要的组分,在长途运输过程中易与区域性人为源污染物发生非均相化学反应,形成组分复杂的混合态颗粒物,从而对气候变化产生更加显著的影响。本研究选择有机胺和粘土矿物分别作为人为源有机污染物和矿物颗粒物的代表,结合理论化学计算和实验方法,系统研究了大气有机胺与硫酸在粘土矿物颗粒物表面的非均相反应机制及其对混合态气溶胶的贡献。研究结果表明,有机胺-硫酸-粘土非均相反应体系通过酸碱中和作用形成富含胺盐的混合态颗粒物,并且这种混合态颗粒物比均相形成的有机胺-硫酸团簇具有更高的稳定性。这表明,当沙尘暴携带大量矿物颗粒物进入富含有机胺人为源的区域时,有机胺-硫酸-粘土混合态颗粒物很可能是气溶胶形成新途径。而有机胺盐具有强吸湿性,将进一步改变混合态颗粒物的云凝结核/冰核的活性,从而对区域性气候产生更加深远的影响。

在沙尘暴期间,大量的矿物颗粒物与人为污染形成比单一矿物颗粒物组分更加复杂的混合态颗粒物,从而对区域性气候产生更加复杂的影响。尽管混合态颗粒物的形成机制受到广泛关注,但是大部分研究涉及的是无机人为污染物在单一组分的矿物颗粒物表面的非均相反应机理。因此,本研究选择了有机胺和粘土矿物分别作为有机污染物和矿物颗粒物的代表,系统研究了有机胺(amine)与硫酸(SA)在高岭石(Kao,粘土矿物主要组分)表面上的非均相反应机制及其在多种大气环境条件下对混合态颗粒物的贡献。结合理论化学计算方法和实验方法,对比研究了Kao-SA-amine和Kao-H2O-SA-amine两种非均相反应体系在有水和无水参与的条件下反应机制的差异。同时,定量评估了两种非均相反应机制对混合态颗粒物的贡献,并且探究了甲基基团数量(1-3),相对湿度(1-100%)以及温度(220-298.5 K)等因素对混合态颗粒物组分的影响。结果表明,水在混合态颗粒物的形成过程中起到关键性作用。具体表现为水的参与能够显著降低胺盐转化的形成能,从而促进有机胺与硫酸在矿物表面发生非均相反应过程。值得注意的是,水的促进效应随着大气环境湿度的升高而增强,而随着大气温度的升高而减弱。对于含有较少甲基基团的甲胺和二甲胺,二者对应的Kao-H2O-SA-amine非均相反应机制是形成混合态颗粒物的主要机制;然而,对于含有更多甲基基团的三甲胺,Kao-SA-amine非均相反应机制是形成混合态颗粒物的主要反应机制。无论经由哪种非均相反应机制形成的混合态颗粒物,胺盐均是最主要的组分。具有强吸湿性的胺盐能够进一步改变混合态颗粒物的吸湿性,而混合态颗粒物通过参与云凝结核和冰核的形成,进一步改变颗粒物的辐射强迫特性,从而对区域性气候产生显著的影响。
论文网址:https://doi.org/10.1016/j.scitotenv.2022.153336
图片摘要
英文摘要:
During dust storm, mineral particle is frequently observed to be mixed with anthropogenic pollutants (APs) and forms mixing particle which arises more complex influences on regional climate than unmixed mineral particle. Even though mixing particle formation mechanism received significant attention recently, most studies focused on the heterogeneous reaction of inorganic APs on single composition of mineral. Here, the heterogeneous reaction mechanism of amine (a proxy of organic APs) with sulfuric acid (SA) on kaolinite (Kao, a proxy of mineral dust), and its contribution to mixing particle formation are investigated under variable atmospheric conditions.Two heterogeneous reactions of Kao-SA-amine and Kao-H2O-SA-amine in absence/presence of water were comparably investigated using combined theoretical and experimental methods, respectively. The contribution from such two heterogeneous reactions to mixing particle formation was evaluated, respectively, exploring the effect of methyl groups (1−3 -CH3), relative humidity (RH) (11−100%) and temperature (220−298.15 K). Water was observed to play a significant role in promoting heterogeneous reaction of amines with SA on Kao surface, reducing formation energy of mixing particle containing ammonium salt converted by SA. Moreover, the promotion effect from water is enhanced with the increasing RH and the decreasing temperature. For methylamine and dimethylamine containing 1-2 -CH3, the heterogeneous reaction of Kao-H2O-SA-amine contributes more to mixing particle formation. However, for trimethylamine containing 3 -CH3, the heterogeneous reaction of Kao-SA-amine is the dominant source to mixing particle formation. For mixing particle generated from the above two heterogeneous reactions, ammoniums salts are supposed to be predominant components which is of strong hygroscopicity and further leads to significant influence on climate by altering radiative forcing of mixed particle and participating in the cloud condensation nuclei and ice nuclei.
资助项目:本研究受到国家自然科学基金(42020104001, 41907184和42007327),广东省基础研究和应用基础研究基金项目(2021A1515011252),广东省本土创新团队项目(2017BT01Z032)的资助。