近日,广东工业大学环境健康与污染控制研究院、环境科学与工程学院高艳蓬教授、安太成教授和加拿大阿尔伯特大学Xing-fang Li教授等在消毒副产物形成机制研究方面取得新进展,研究成果以《Formation Mechanism of Iodinated Aromatic Disinfection Byproducts: Acid Catalysis with H2OI+为题发表在Environmental Science & Technology(2022, 56(3): 1791–1800)杂志上。该研究重点针对高毒性碘化消毒副产物(I-DBPs)形成机制不明晰的问题,采用量子化学计算与实验研究相结合的研究手段,从分子水平阐明了I-DBPs形成过程中H2OI+酸催化作用,为减少毒害消毒副产物I-DBPs暴露和健康影响提供了新见解。
第一作者: 高艳蓬 教授 邱俊琅 博士
通讯作者: 安太成 教授 Xing-fang Li 教授
通讯单位: 广东工业大学 阿尔伯塔大学
论文DOI: https://doi.org/10.1021/acs.est.1c05484
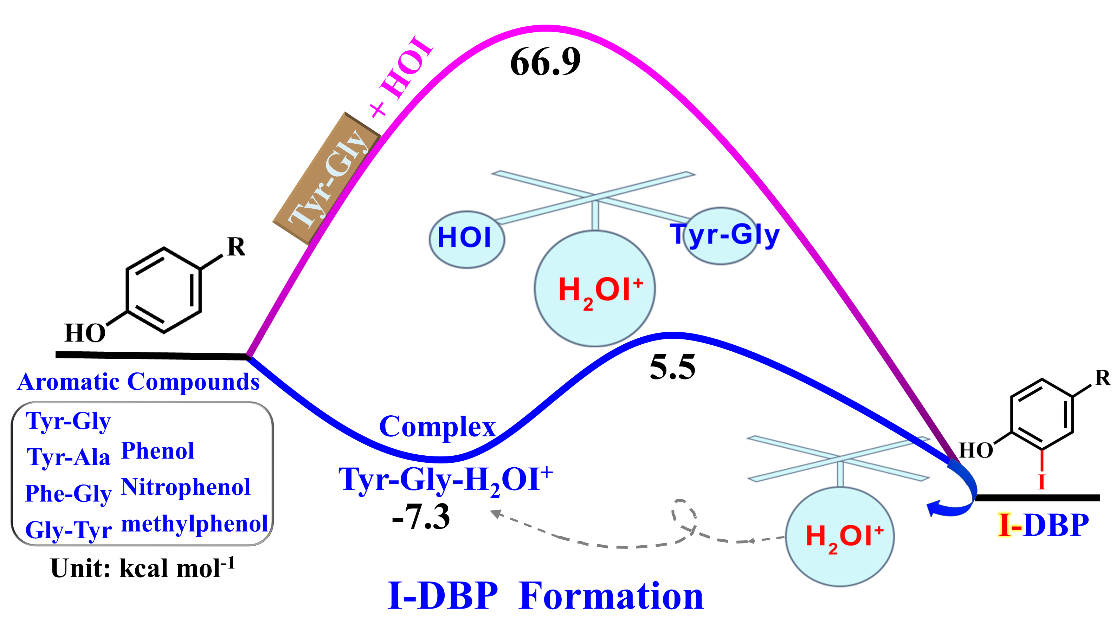
碘代消毒副产物(I-DBPs)是一类高毒性、且尚未被监管的消毒副产物。由于含碘的氯胺化水中存在大量HOI,因此人们通常将I-DBPs的形成归功于HOI反应。在本研究中我们首先采用量子化学计算研究了芳香类污染物的碘化机理,如二肽和酚。结果表明碘化产物(I-Tyr-Gly)比羟基化产物(OH-Tyr-Gly)形成需要经历更高的反应能垒,这说明HOI与芳香类化合物反应将更容易形成OH-Tyr-Gly,而并非I-Tyr-Gly。意外的是,实验中高分辨质谱数据中仅可以找到I-Tyr-Gly,而没有OH-Tyr-Gly。实验与理论研究结果的明显分歧说明了I-Tyr-Gly的形成不能简单地仅仅归因于HOI反应,还可能存在其它未知机理。为了进一步深入揭示这一科学问题,作者继续研究了体系中的其它碘化试剂的贡献,计算结果表明H2OI+与芳香类化合物可以自发形成络合物,这可能是启动I-DBPs形成过程的关键机制。当H2OI+作为酸催化剂和碘化剂,HOI或H2O作为质子受体时,I-DBPs形成具有较低的反应能垒(仅需要10.8-13.1 kcal mol-1),同时释放出一分子H2OI+,也就是说H2OI+在这个过程是不消耗的,即一分子的H2OI+参加反应,反应后又形成了一分子的H2OI+,即H2OI+在反应过程中起到类催化剂的作用。因此即使在水环境中仅存在非常微量的H2OI+,也是可以不断地参与I-DBPs的形成过程。该研究结果揭示了芳香类I-DBPs形成的新机理,为饮用水中I-DBPs的控制研究提供了非常宝贵的有用信息。
论文网址: https://doi.org/10.1021/acs.est.1c05484
图片摘要:
论文的英文摘要附如下:
ABSTRACT:
Iodinated aromatic disinfection byproducts (I-DBPs) are a group of nonregulated but highly toxic DBPs. Formation of I-DBPs is attributed mainly to HOI because it is the most abundant reactive iodine species in chloraminated water. In this study, we used computational modeling of thermodynamics to examine the mechanism of iodination of aromatic contaminants, e.g., dipeptides and phenols. Computational prediction of the energy barriers of the formation of iodinated tyrosylglycine (I-Tyr-Gly) (66.9 kcal mol-1) and hydroxylated Tyr-Gly (OH-Tyr-Gly) (46.0 kcal mol-1) via iodination with HOI favors the formation of OH-Tyr-Gly over I-Tyr-Gly. Unexpectedly, mass spectrometry experiments detected I-Tyr-Gly but not OH-Tyr-Gly, suggesting that I-Tyr-Gly formation cannot be attributed to HOI only. To clarify this question, we examined the thermodynamic role of the most reactive iodine species H2OI+ in the formation of aromatic I-DBPs under chloramination. Computational modeling of thermodynamics results shows that a loosely bonded complex formation of aromatic compounds with H2OI+ is the key step to initiate the iodination process. When H2OI+ serves as acid-catalyst and iodinating agent, with HOI or H2O acting as proton acceptor, the energy barrier of I-DBP formation was significantly lower (10.8-13.1 kcal mol-1). Therefore, even with its low concentration H2OI+ can involve in the formation of I-DBPs. These results provide insight into the mechanisms of aromatic I-DBPs formation and important information for guiding research toward controlling I-DBPs in drinking water.