近日,广东工业大学环境健康与污染控制研究院、环境科学与工程学院安太成教授团队题为《The respiratory cytotoxicity of typical organophosphorus flame retardants on five different respiratory tract cells: Which are the most sensitive one?》的学术论文在国际期刊Environmental Pollution(2022.307: 119564)杂志上发表。论文第一作者为硕士生陈静漪,通讯作者为安太成教授。该研究主要关注了五种自上而下的不同人体呼吸道细胞在典型有机磷阻燃剂(OPFRs)磷酸三苯酯(TPHP)暴露下的毒性水平和致毒趋向,探讨了不同细胞是否启动相似或者不同的死亡或者应激机制,模拟真实人体呼吸系统的环境暴露水平下的OPFRs的内质网应激,氧化应激和线粒体损伤机制,以及比较不同细胞解毒方式和应激途径的差异,包括靶点细胞的信号通路富集情况等。为OPFRs在人体呼吸道的暴露作用靶点给出更确切的实验依据。该研究对真实环境情况下OPFRs呼吸道暴露后导致呼吸道相关疾病产生和预防提供了更详细的信息。
论文DOI:https://doi.org/10.1016/j.envpol.2022.119564
磷酸三苯酯TPHP是一种广泛使用的阻燃剂,通常在生活消费品中用作增塑剂,在商用发动机油中用作润滑剂。2007年中国有机磷阻燃剂(OPFRs)的产量预计为7万吨,2019年增加到10,500吨,生产量和使用量逐年递增。迄今为止,OPFRs在动物和人类中的累积效应及其生物转化行为已经成为全球热点问题。然而,这些研究还没有得到全面的总结和归纳。对OPFRs的吸收、生物积累和代谢转化过程的研究将有助于解释OPFRs的命运和毒性机制。因此,迫切需要在系统的框架内对现有的环境分布特征进行研究,以便更好地了解OPFRs在生物体中的积累,转化,代谢的行为和命运。
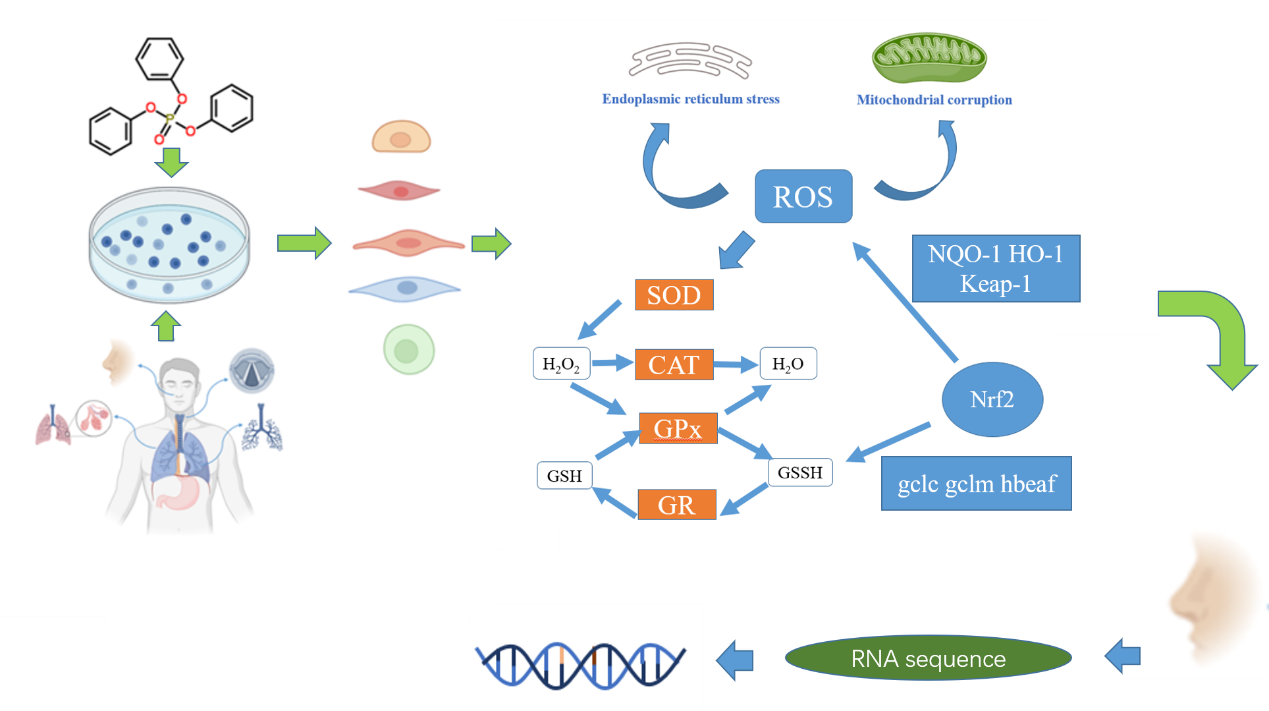
本研究通过典型OPFRs的暴露结果表明:肺纤维细胞HFL1和鼻粘膜上皮细胞HNEpC细胞被发现是不可逆的损伤,而其他三种细胞,如人支气管上皮细胞16HBE,肺上皮细胞Bes-2B和人体咽喉细胞NP-69则通过ATF4内质网应激总开关,被早期内质网应激拯救机制激活,Ca2+外流明显,实现了短期自我修复。
随着暴露程度加重,不同细胞抵御TPHP的阈值出现差异。当内质网应激无法缓解TPHP带来的损伤,则启动后期活性氧ROS转导的线粒体的凋亡通路。我们发现受损最严重的是HNEpC细胞,其中Nrf2信号通路以及下游基因表达上调1.3 ~ 7.0倍,2 ~ 10 (U/mg protein)和谷胱甘肽解毒酶活性改变。起初细胞受到TPHP刺激后,诱导细胞转录子XBPI发生可变剪切,磷酸化后促进转录子ATF4翻译,形成活跃转录子,启动内质网应激,应激诱导ROS生成,产生相关炎症因子和趋化因子。同时诱导Nrf2蛋白从keap-1解离,转录进入细胞核中,产生谷胱甘肽催化亚基(gclc),修饰亚基(gclm)生成,启动谷胱甘肽还原系统,生成谷胱甘肽,从而抵御对细胞的损伤。此外,与慢性鼻窦炎发病相关的血管内皮生长因子(VEGF)在HNEpC细胞中增加了1.0 - 3.5倍。
这是第一篇通过OPFRs暴露下整个人呼吸道的不同呼吸细胞毒性的比较研究,发现HNEpC细胞是TPHP最敏感的靶点。最后通过RNA测序结果表明:典型TPHP暴露对HNEpC的蛋白连锁重组、分子功能调控和代谢过程信号通路均有影响。并且基于KEGG和GO数据库的分子生物学研究揭示了HNEpC暴露TPHP可诱导MAPK信号通路的激活。
论文网址:
https://www.sciencedirect.com/science/article/abs/pii/S0269749122007783?via%3Dihub
图文摘要
论文的英文摘要附如下:
ABSTRACT:
Triphenyl phosphate (TPHP) is a frequently used flame retardant and indoor semi-volatile pollutant exposing humans with endocrinal disrupting effects. However, its respiratory tract toxicity remains unclear. Herein, we mainly focused on exploring the cytotoxicity of TPHP to the cells from five different parts of the human respiratory tract (from top to bottom): human nasal epithelial (HNEpC) cells, human bronchial epithelial (16HBE) cells, normal nasopharyngeal epithelial (NP69) cells, human lung epithelial cells (Beas-2B) cells, and human lung fibrocells (HFL1 cells) cells. The cell viability, micronucleus induction, endoplasmic reticulum stress gene, intracellular Ca2+concentration, mitochondrial membrane potential (MMP) were investigated in short-term as well as extended exposure of TPHP. HFL1 and HNEpC cells were found to be irreversible damage, while other three type cells achieved homeostasis through self-rescue. Moreover, expression of downstream genes of Nrf2 signaling pathway were upregulated for 1.3 – 7.0 times and glutathione detoxification enzyme activity changed for 2 – 10 (U/mg protein) in HNEpC cells. Furthermore, the vascular endothelial growth factor (VEGF), a disease-related factor, increased 1.0 – 3.5-fold in HNEpC cells. RNA-sequencing results suggested that protein linkage recombination, molecular function regulation and metabolic processes signal pathway were all affected by TPHP exposure in HNEpC. This is a first report to compare respiratory cytotoxicity in whole human respiratory tract under OPFRs exposure and found HNEpC cells were the most sensitive target of TPHP. Molecular biological mechanisms uncovered that TPHP exposure in HNEpC can induce the activation of MAPK signal pathway and demonstrate potential respiratory growth differentiation and stress disorder in human nasal cells upon TPHP exposure.